CASE STUDY: STERIFRE AURA
![]()
An EPA Registered, Automated, Point-of-Care Disinfection Device to Help Combat COVID-19 and other Healthcare Associated Pathogens
Sterifre Medical had an initial design for their AURA product, an automated point-of-care disinfection device, when they selected Simplexity as their engineering development partner to refine the design. AURA takes a novel, patented approach to device disinfection for the medical device industry with the simple press of a button. It is the first automated, point-of-care device that can disinfect handheld diagnostic and therapeutic non-critical devices from stethoscopes to sensors, meters, pumps, and communications devices. Even staff and patient personal items like cell phones, keys, and badges can be safely and effectively disinfected. AURA offers broad material compatibility with broad-spectrum efficacy with no chemical residue, device damage, environmental impact, or employee chemical exposure experienced with currently available disinfectant wipes and sprays. Unlike UV systems that are unregulated and offer an incomplete solution, AURA is a fully regulated hospital disinfection device that has been approved by the EPA.
Introducing Sterifre AURA
THE CHALLENGE
Sterifre selected Simplexity to refine the design of their AURA system to meet important criteria for product launch while continuing to follow a list of strict product requirements. Simplexity used a phased approach to achieve this goal.
- In the first phase completed, the Simplexity team conducted an in-depth product assessment. The team established a viable path forward to reduce the system’s noise level by approximately 10 dBA, reduce system weight roughly in half, and deliver a system at a cost consistent with Sterifre’s manufacturing cost goals.
- The Simplexity team completed the detailed refinements, and assembled a small number of AURA units, implementing these refinements. These units were used to confirm that the design met all product requirements and matched manufacturability goals before committing to tooling.
- Once through architecture of the new product, the Simplexity’s NPI team helped Sterifre identify the best contract manufacturer for their product based on major subsystem components, production schedule goals, and cost targets.
- In the current phase Simplexity is working closely with the contract manufacturer and their suppliers to further optimize the design for manufacturability, make minor corrections to individual parts to optimize the assembly process, fabricate the tooling for all custom parts, and complete the design validation build and testing prior to product launch. Outside of the instrument development, the disposable fluid cartridge required a separate CM with parallel planning activities to ensure integration into the overall instrument schedule.
The initial commercial design had several major areas that were critical to address to meet Sterifre’s goals. These were:
- Reduced Noise: The initial units were considered too loud at 45-50 dBA, the refined system is significantly quieter and much more consistent.
- Weight: AURA weighed in at a hefty 18.3 kg. The refined AURA systems weigh 11.1 kg with convenient carrying handles molded into the case.
- Cost: The cost to manufacture the units needed to be lowered by 38% to meet manufacturing cost goals.
These were challenging targets that would require Simplexity’s multi-disciplinary team of engineering experts to develop creative solutions and take a disciplined approach in analyzing and implementing refinements prior to scaled production ramp.
 |
 |
 |
“We appreciate the depth of talent of the Simplexity team. Some of the other folks we looked at just do medical products. We didn't view that as crucial for us. What was important to us was that the talent was there to tackle the job no matter what the engineering issue was. Drawing from their experience on many different products, not just medical, is a big plus. We went through our check list of attributes, and I believe we picked a winner with Simplexity.”
-Rick Shea,
CEO at Sterifre Medical
Noise Reduction
To determine potential for noise reduction improvements, one of the initial AURA systems was converted into a Noise Breadboard. The amount of noise generated at 1 meter from the unit was measured as a baseline. The team took a systems approach to brainstorming ideas for reducing the noise of the unit. Then each idea was prototyped and the effect on noise was measured.
Based on this analysis and testing, the following modifications were implemented to reduce system noise:
- Line the case parts with acoustic insulation.
- Use a minimum ratio of 2 ½ inlet diameters of smooth ducting leading to the inlet of the blower.
- Add a muffler on the blower outlet.
- Mount the blower using vibration damping grommets between the mounting bracket and the frame.
- Block off bulk access between the airflow through the system and the exhaust filter, replacing it instead with a thin connection such as a length of tubing to maintain the desired pressure in the system.
- Reduce flow restrictions in the ducting and key sub-assemblies to enable running the blower at lower speeds while achieving the desired airflow through the system.
By incorporating the six modifications described above, the resulting sound pressure was decreased by over 10 dBA and sound quality is now deemed acceptable for the application.
Weight Reduction
As a point of care system, the weight of the Sterifre AURA system is important. Too heavy of a device will make handling and transport cumbersome. The initial AURA design was roughly twice as heavy as the product specification goal.
Simplexity started the weight analysis by creating a Pareto chart, categorizing which components had the greatest weight contributions. This resulted in understanding that much of the weight is made up of molded plastic or sheet metal components. The case parts, airflow system, and chamber make up nearly 80% of the mass of the system, so much of the effort to design a lighter system focused on those subassemblies.
Case Parts
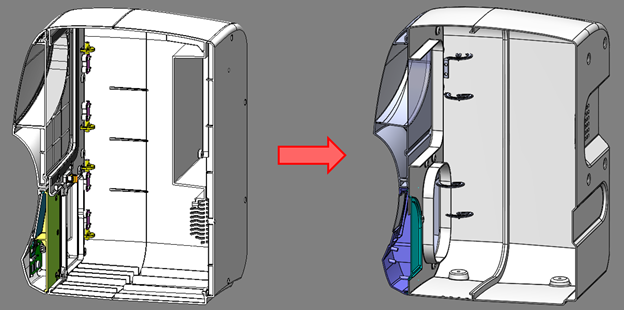
Mass was reduced in the case parts by reducing wall thickness on the case and doors. The nominal wall thickness was reduced from 3 mm to 1.5-1.8 mm while still maintaining structural rigidity. The back panel was integrated into the main case, eliminating a double layer of case parts on the back of the unit.
Figure 5: Original AURA vs. refined AURA Case Part Design Simplification
Airflow System
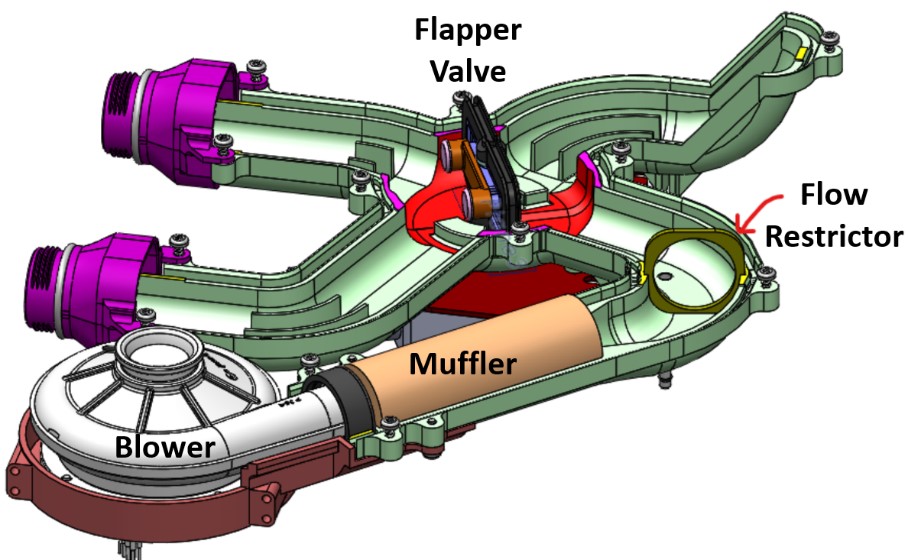
The refined AURA system substantially simplified airflow. The four 2/2 flapper valves from the original AURA design were consolidated into one 4/2 and one 3/2 butterfly valve and integrated into the ducting. The duct walls were also be reduced in thickness which will lead to additional weight savings.
In order to diagnose any potential blower issues, an orifice plate was added to the ducting between the muffler outlet and the 4/2 valve, with pressure taps on either side. The orifice plate constricts flow through the ducting, creating a pressure drop across it. By measuring the pressure differential across the taps located just upstream and downstream of the orifice plate, the flow rate through the system can be measured.
Figure 6: 4/2 manifold assembly featuring the blower, muffler and flapper valve
Support Structure
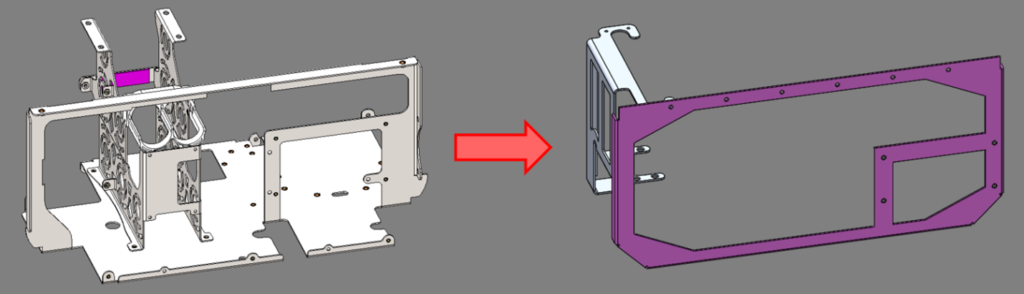
The main structure in the initial design was a stainless steel sheet metal bracket that weighed 1.3 kg (2.9 lb). By mounting the subassemblies directly to the chamber in the refined design, this panel was removed and replaced by two smaller aluminum sheet metal parts, weighing a combined 0.3 kg (0.7 lb). This resulted in a 77% weight reduction for this subassembly.
Original stainless steel support structure vs. optimized aluminum brackets
Chamber
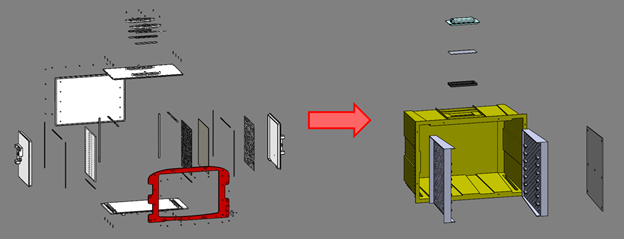
The chamber was a major area of opportunity for mass reduction. The body of the chamber in the initial design consisted of 6 panels, each nominally 6.35mm thick. This was reduced to one larger part with a nominal 1.5mm wall thickness. This substantial reduction in wall thicknesses in this subassembly saved 2.4 kg (5.3 lb) of weight.
Original vs. refined chamber design
Total Weight Savings
The refined design reduced the weight from 18.3 kg to 11.1 kg (24.5 lbs), a 40% weight savings.
Cost Reduction
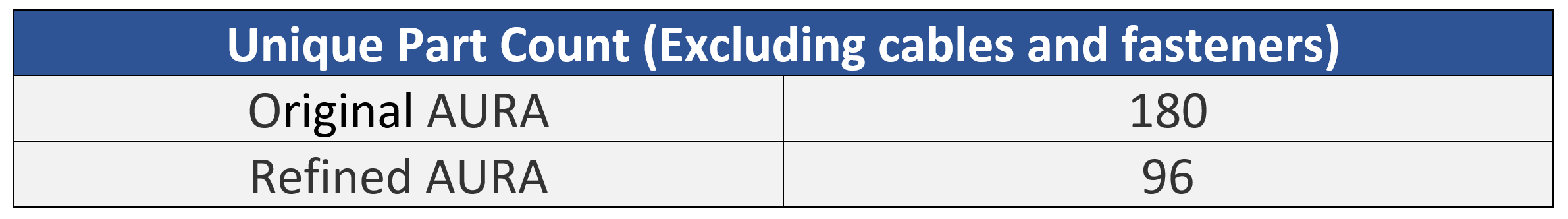
One of the first tactics employed in the cost reduction activity was to analyze the entire Bill of Materials in the original design to remove, combine, and/or simplify parts. Eliminating unnecessary parts is more effective than trying to make a part cheaper to manufacture. An example of one of the cost reductions recommended was to decrease the chamber body from 10 custom parts (7 unique) to 1 part designed for injection molding. Another example was replacing an expensive bearing linear guide with simple, inexpensive parts engineered to perform the same function at a fraction of the cost.
Based on the cost reduction analysis and optimized design the team reduced the total part count by 47%, from 180 to 96 unique parts.
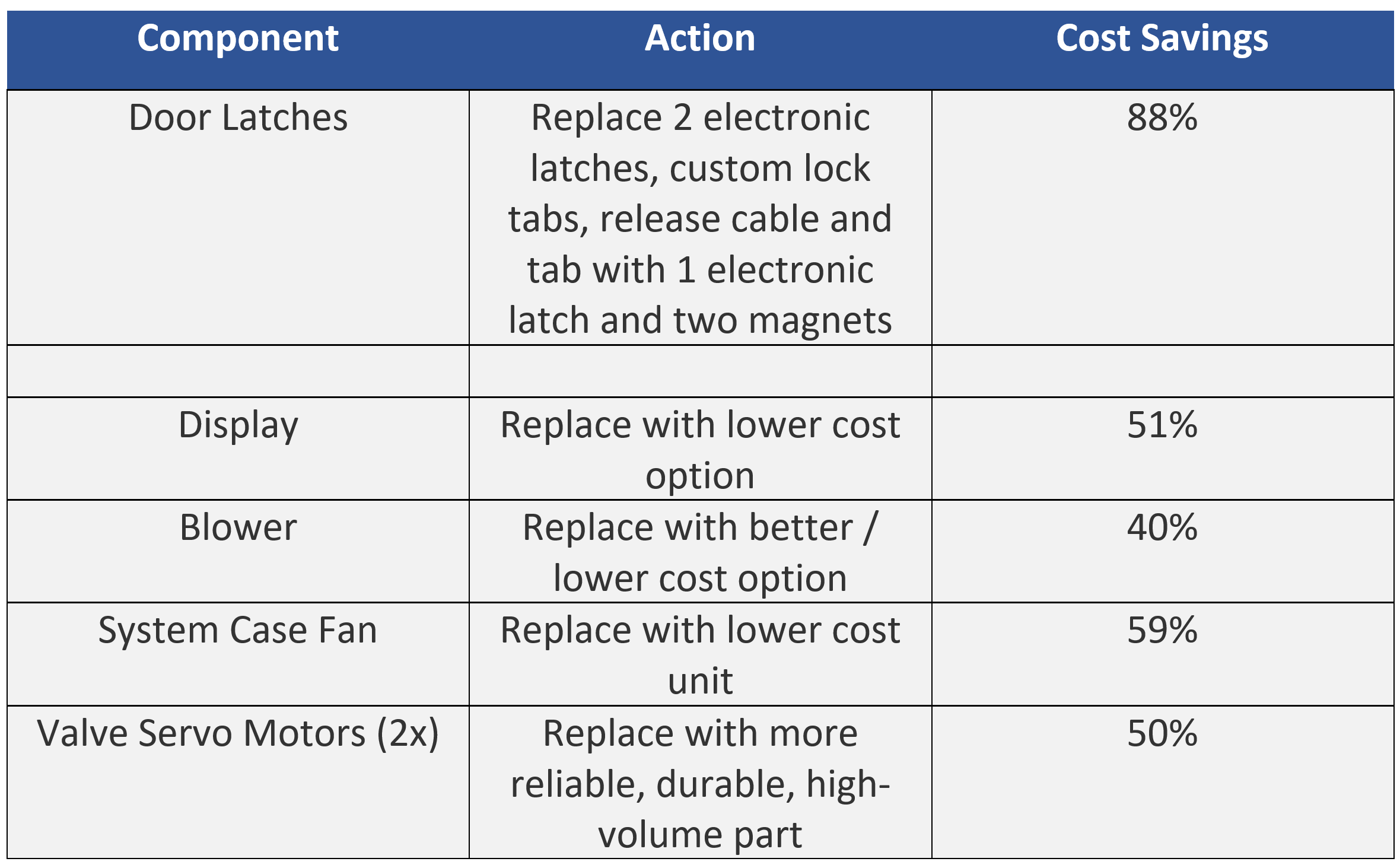
Another key tactic to cost reduction is evaluating and replacing off-the-shelf components with lower cost parts that meet the technical specifications. Examples of component level cost reduction are listed below.
Implementing these changes presented distinct challenges. For example:
- Identifying lower cost blowers to circulate the effluent mixture through the machine leveraged existing supply chain options for CPAP blowers where component prices vary by 3x. The range of suppliers who were willing to ensure chemical compatibility for this application using strong oxidizing agents significantly reduced the supplier options. The solution was to select an existing blower that featured sealed bearings and motor and change the impeller material from nylon to PEEK. The resulting blower is much more reliable, can move the required airflow, and is much lower cost.
On the electrical engineering side, the entire printed circuit board set was re-designed from the ground up, while still meeting product specifications and safety requirements. Key changes included:
- Merging two MCUs into one MCU
- Utilizing a semi-custom 4000-volt power supply for activated oxygen generation.
- Designing an intermediate power supply between line voltage and the high voltage supply enabled better control of activated oxygen generation. It also allows for 120-volt or 240-volt operation.
- Incorporated custom circuitries and combined them into fewer boards to drive valve motors, read sensors, actuate nebulizer, and meter precise quantities of H2O2.
- Utilizing an off-the-shelf medical grade power supply for system voltages
- Minimizing unnecessary redundancies
The scope of this project also included Simplexity writing the embedded firmware to control, run, and monitor performance of the device. While lines of code are not as easy to visualize from a cost reduction standpoint, they do affect the size of processor that was needed. Thus, the firmware architecture was optimized to require as small a processor as possible.
Continued Cost Reduction Efforts
The actions mentioned above are a good start, but even though we have built units for engineering confidence testing, we are not done driving down cost yet. Simplexity has expertise in design, but we also tap into the expertise, knowledge, and experiences of the contract manufacturer as a full partner to continue to lower manufacturing costs.
Simplexity recently hosted the contract manufacturing (CM) technical staff at our facility to participate in fully assembling an AURA unit. This exercise provided the manufacturing experts who will be responsible for the product in scaled production the opportunity to have hands-on experience with the product. The team also brainstormed improvements in Design for Manufacturability (DFM) of each component and improvements in Design for Assembly and Test (DFA / DFT). This also allowed the teams to organize an actionable list of opportunities to reduce component cost, and assembly time. The exercise produced a punch-list of over 200 actionable suggestions for minor changes that will improve manufacturability and reduce costs. Additionally, it sets the stage for the CM to facilitate the DFM conversations with their supply chain partners to help reduce part cost and tooling cost within the context of design intent.
Simplexity's New Product Introduction (NPI) Role
Some of Simplexity’s New Product Introduction contributions on the project include:
- Once through architecture of the new product, Simplexity took on the role of identifying the best fit CMs for further evaluation
- Sharing of major subsystem components, production schedule goals, and cost targets allowed for further down-selection to a single CM
- Once the CM was selected, additional discussions and planning were performed to provide a detailed cost and timeline for initial product ramp
- Of key importance were tooling lead times and costs, as this was critical to Sterifre’s funding milestones
- Outside of the instrument development, the disposable fluid cartridge required a separate CM with parallel planning activities to ensure integration into the overall instrument schedule
“Simplexity has a very disciplined, systematic approach in the way they work. They provide us with options for solutions and make sure we understand trade-offs as we make decisions. All of these things come together to meet our design challenges.”
-Gen Minor,
VP of Quality & Regulatory Affairs at Sterifre Medical
Sterifre AURA Customer Testimonial
This case study exemplifies the methodology and technical expertise that Simplexity can bring to bare in a complex, highly regulated, product engineering scenario. Certain aspects of the program, not outlined in this case study, involve highly proprietary product developments which are not discussed herein as they are the subject of patent applications. Simplexity is accustomed to working with clients with proprietary intellectual property.