CASE STUDY: OREGON HEALTH & SCIENCE UNIVERSITY (OHSU) VASCULAR MONITORING
Exploring how to improve the experience of vascular monitoring via functional prototypes of ultrasonic sensors and applicators.
The Challenge
Constant monitoring of vascular activity in surgery patients is critical in assessing patient health. It is customary practice to use an ultrasonic-based sensor to evaluate and establish proper vascular activity.
Oregon Health & Science University’s (OHSU) Surgical Innovation Program asked Simplexity for concepts and prototypes for a vascular monitoring device that replaces a traditional bandaged sensor that requires constant repositioning and interaction from highly trained staff.
Fundamentally, the challenge was to increase patient comfort and reduce interaction by staff while improving vascular activity monitoring. Simply put – can we design a sensor housing that increases patient comfort, reduces expensive staff interactions, and improves patient data? Can we develop intellectual property (IP) that OHSU can spin off or license?
Simplexity accepted the challenge.
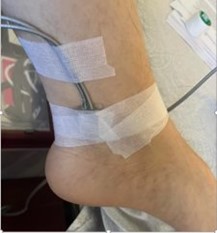
Original application of ultrasonic sensor – with low level of patient comfort and requiring
elevated level of staff interaction.
The Development
The first phase of development was to dive deep with feedback from OHSU managers and surgical doctors to understand the root of the issues from the current ultrasonic sensor use model. During our first brainstorming session, it was clear there were vast opportunities to improve the application, attachment method, and usability of the sensor. Not only could we improve the physical attachment of the sensor to the patient, but via thoughtful design – we could find a solution that would improve the use model and integration process by highly trained staff.
Using fast paced and highly collaborative team meetings – we were able to iterate quickly and efficiently and flesh out the most valuable design features. With the aid of in-house 3D printing and a choiceful integration of off the shelf (OTS) adhesives, we quickly cycled through 15+ unique physical iterations of the applicator.
From desktop testing and collaborative discussion – the team down-selected to 2 top applicator concepts that were created, packaged, and provided to OHSU physicians for multi-day testing and evaluation. The evaluation of the 2 prototypes (multiple copies of each) was successful – meeting or exceeding the requirements noted below.
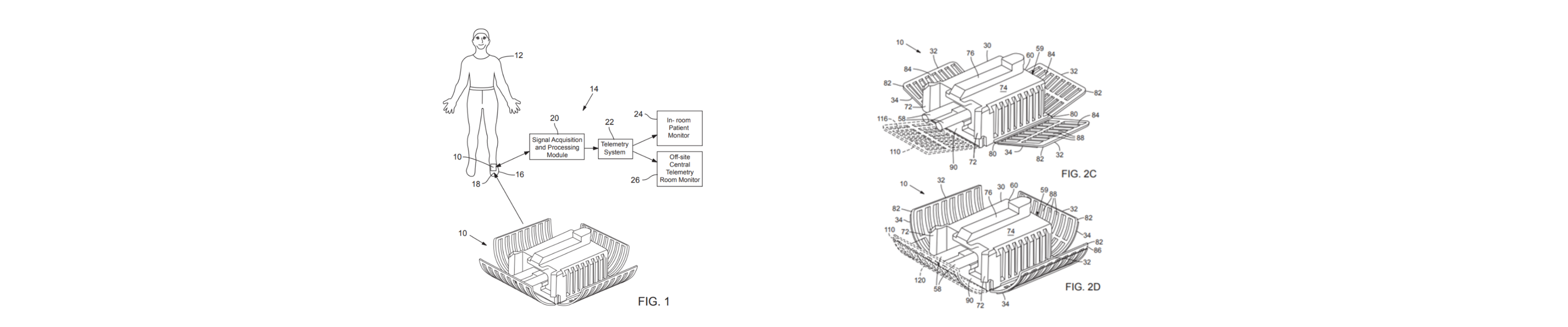
With the success of the informal prototype trials by OHSU physicians, the OHSU Surgical Innovation Group asked the OHSU Technical Transfer team to submit a patent application that covered the wide array of features developed in the development process.
The Requirements
The sensor housing (“applicator”) had the following basic requirements –
- Must be safe and comfortable to wear for 48hrs – even on mobile/walking patients.
- Must enable successful ultrasonic vascular monitoring with minimal interaction.
- Must be able to be applied at ankle and top of foot, both on the left and right
- Must protect and support aqueous gel pad for sustained ultrasonic measurements.
- Must significantly reduce MD interaction with patient for routine device readings.
- The design solution must be novel enough to yield licensable IP for the OHSU Innovation group.
Product Design & Engineering
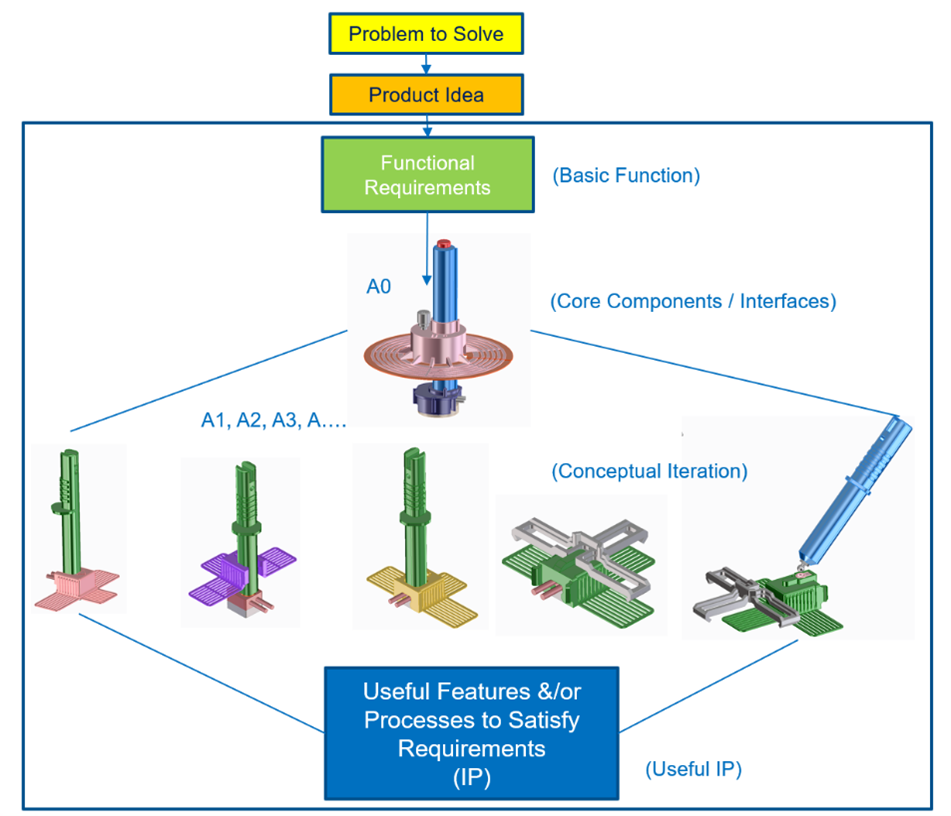
Phase 0
Phase 0 was all about understanding the product use model, finding solutions to functional requirements, and rapid evaluation of concepts via focused discussions and meetings. The team pinned down critical functional requirements that would support an improved use case (for staff during application and monitoring) and an improved patient experience (during “wearing” the sensor). Furthermore, via this conceptual phase, we established the minimally viable product features required for a startup in the productization of this applicator design.
Phase 0 path to discovering design utility.
Phase 1
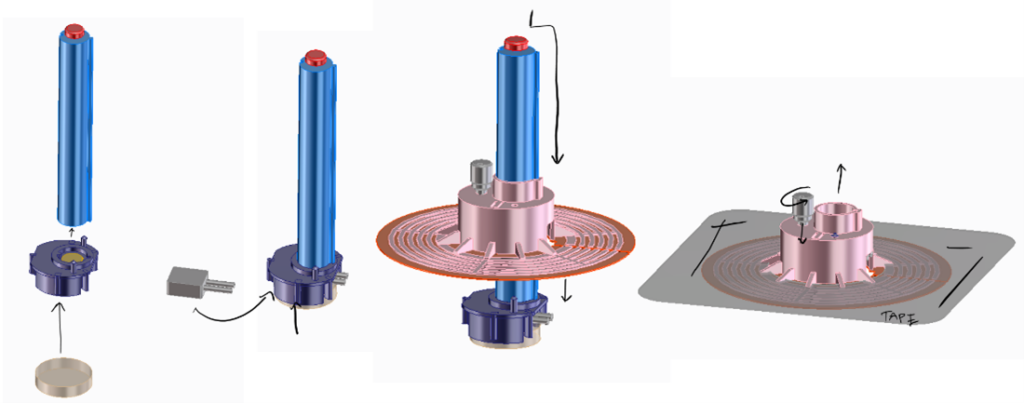
The goal of Phase 1 was to develop quick prototypes to, 1) either build upon or 2) eliminate confidence that we were moving in the correct direction for a physical embodiment of a discrete design. Knowing when to eliminate a concept is just as valuable as discovering utility in a good concept. Knowing when to stop is as important as identifying a clever idea.
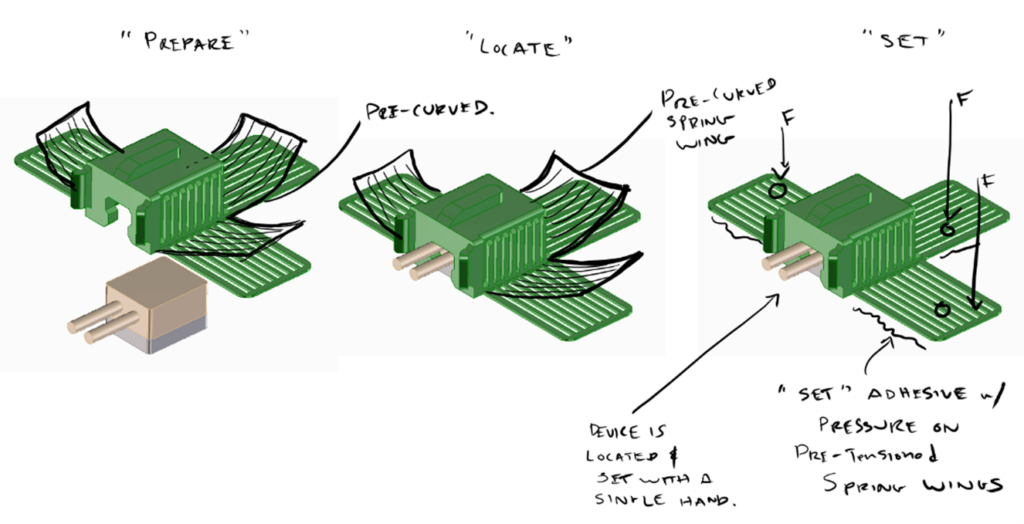
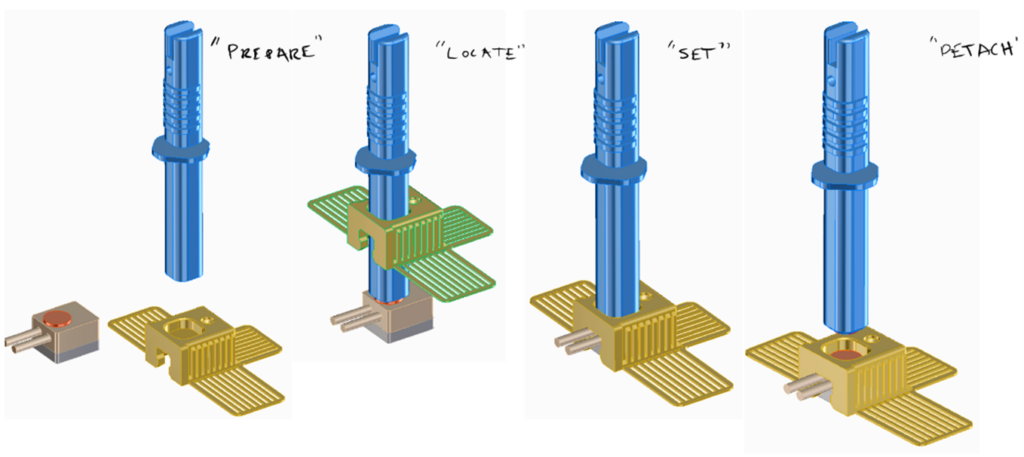
Various design solutions for application and sensor retention were tested. Among many other ideas – these included a “pen” type applicator, curved or flat adhesive application “wings” and multi-polymer “tension claw” features for gel-pad retention.
Conceptual ideas for “pen” type applications.
Conceptual ideas for curved or flat adhesive “wings”.
Conceptual ideas for “magnetic pen” applicator.
Conceptual ideas for “tension claw” aqueous gel pad retention.
Phase 2
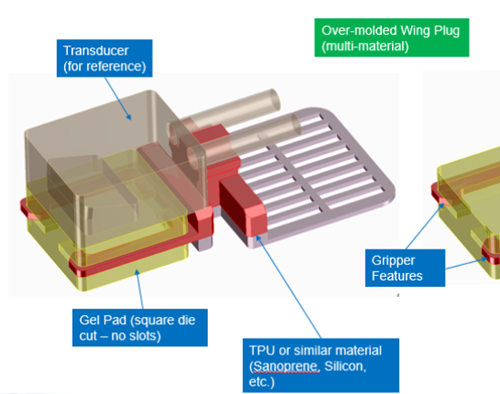
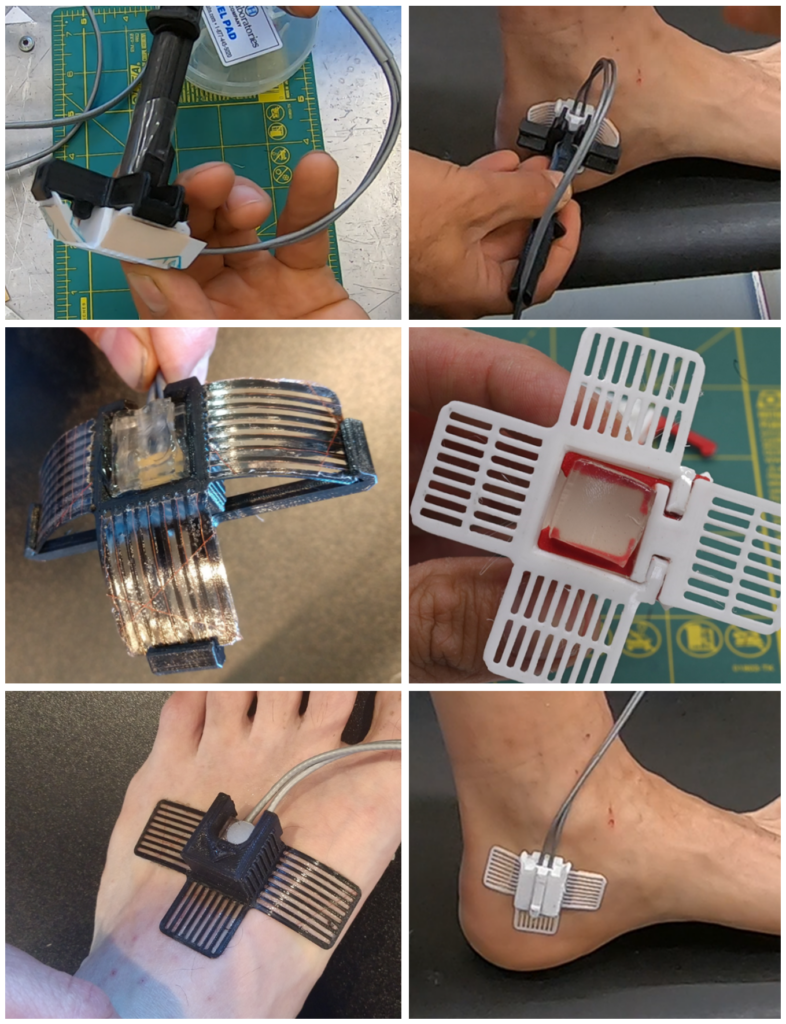
In Phase 2, we used our learnings and experience from Phase 1 to add further detail into the most promising discrete applicator embodiments. Substantial effort was put into adding as much design detail as possible and careful fabrication of each functional prototype. A handful of these functional prototypes would give direct insight to how a larger scale clinical trial could go.
Upon testing and evaluating the numerous functional prototypes – OHSU gained confidence that these design solutions added an elevated level of value to patient care while simultaneously reducing staff interactions. The OHSU Innovation team moved forward with IP submission and a broader internal OHSU evaluation to gain further internal funding.
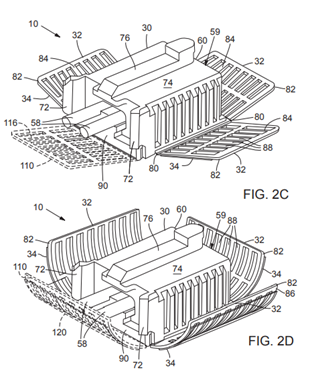
and novel features to pursue patent protection. Various novel features tested and evaluated
were submitted for US Patent Protection.
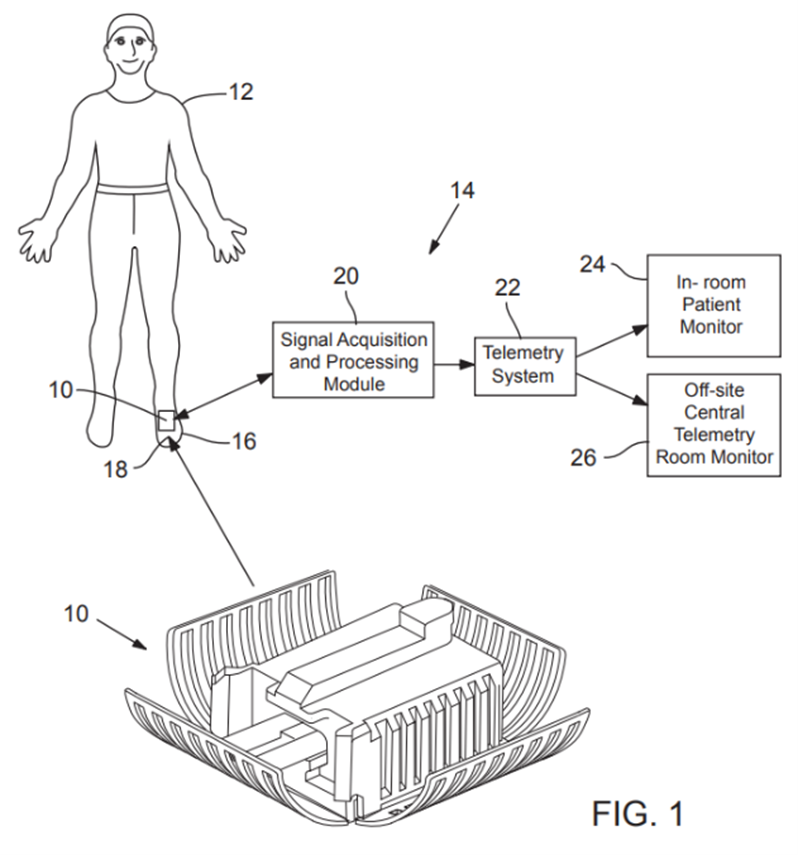
Examples of OHSU submitted drawings for patent protection.
What’s Next?
This technology has been patented and is available for licensing. OHSU looks forward to supporting a unique startup that would license the IP or license it directly to established partners.
Resources
- OHSU Innovates – OHSU # 2951 — Device for Sustained Doppler Telemetry
- Ultrasound and Vascular Lab | OHSU