CASE STUDY: ANAVASI DIAGNOSTICS
MOLECULAR DETECTOR
![]()
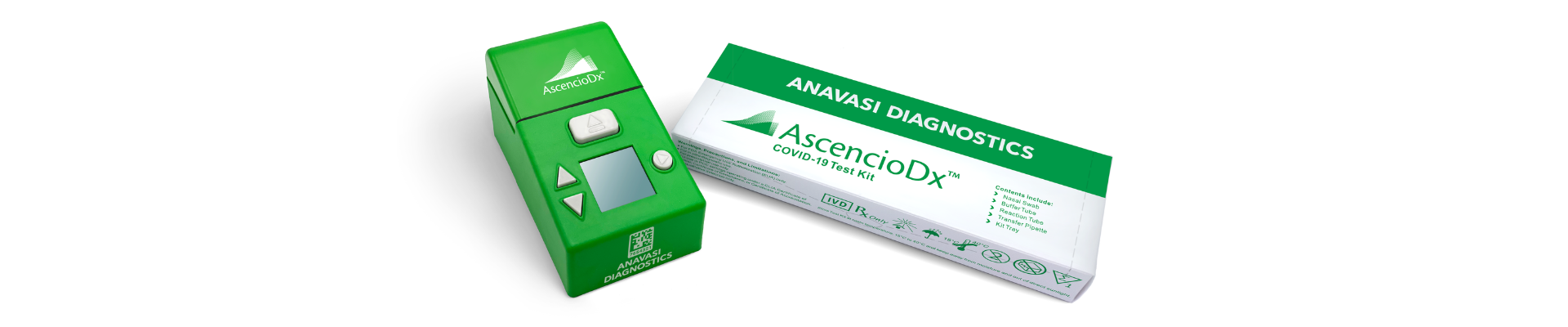
The AscencioDx® COVID-19 Test and The AscencioDx® Molecular Detector is a rapid, highly accurate, easy-to-use, and affordable clinical diagnostic platform used for performing molecular analysis of samples collected from patients in point-of-care (POC) settings
THE CHALLENGE
Molecular diagnostics is the standard for accurate virus detection, but the services and equipment used are costly and often limited to dedicated laboratory settings. When Anavasi Diagnostics needed a partner to help them develop their COVID-19 molecular detector for an easy-to-use, highly accurate, and less expensive option than other molecular (RNA) technology on the market, they turned to Simplexity. Anavasi Diagnostics chose Simplexity to accelerate and de-risk their product development by utilizing a partner who already had complete system design expertise in mechanical, electrical, and firmware engineering. The goal was to preserve the industrial design while addressing functionality, cost, design margin, material compatibility, and design for manufacturing. Simplexity’s product development expertise and NPI (New Product Introduction) services helped Anavasi reduce the project timeline from concept to production transfer.
Anavasi Diagnostics AscencioDx®
THE SOLUTION
Simplexity worked with Anavasi Diagnostics from requirements alignment through detailed design to production transfer. An aggressive schedule was critical to meet the urgent need for this diagnostic platform in a dynamic COVID-19 environment.
Prototypes were designed, built, and delivered in stages, allowing Anavasi Diagnostics to perform testing and share feedback for honing the design quickly. Throughout the process, the design team implemented changes to keep improving the product for simplicity, ease of use, and manufacturability. In addition, Simplexity’s New Product Introduction (NPI) team engaged directly with the chosen contract manufacturer, to develop work instructions, production fixtures, and production line layout.
In parallel, Simplexity also designed, built, and delivered multiple tools for use at Anavasi Diagnostics’ manufacturing partner and parts suppliers. EOL (End-of Line), IQC (Incoming Quality Control), tube leak, and tube opacity testing tools were crucial elements to production success.
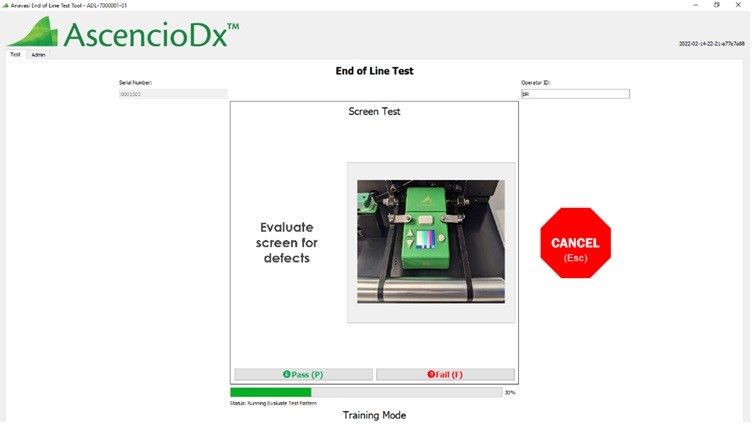
Anavasi End of Line Tool
“Simplexity supported Anavasi Diagnostics with detector development and production transfer which enabled the core Anavasi team to focus on the assay development and commercialization planning. This model enabled significant schedule reduction and lower development costs.”
-Minh Duong, Co-founder, and Chief Engineer at Anavasi Diagnostics
THE RESULTS
With a strategic partner in Simplexity, Anavasi Diagnostics was able to develop and reach production far sooner and at less cost than if their efforts were spread across the development of both the detector and the assay. Simplexity’s Test team executed both component level and system level testing, including verification testing, characterization testing, and regulatory testing performed at area test houses. This team worked diligently to support Anavasi Diagnostics in earning their Emergency Use Authorization (EUA) from the FDA for the AscencioDx COVID-19 Test and the AscencioDx Molecular Detector. Simplexity provided comprehensive experience and expertise throughout the entire development cycle until successful production manufacturing ramp.
To create an easy customer experience, the user can scan the QR code on the device with their smartphone to report results via the Graphical User Interface (GUI).
Both Simplexity and Anavasi Diagnostics believe in a safer, healthier world by providing greater access to tools that provide more accurate detection of viral infection at an affordable price point. Read more about Anavasi Diagnostics and how they are putting the functionality of a whole PCR lab into this simple device that Simplexity helped design and develop. No more waiting for days to get an accurate Covid test result - soon it will be available in as little as 20 minutes.
RESOURCES
- Anavasi Diagnostics Receives FDA Emergency Use Authorization for The AscencioDx COVID-19 Test and The AscencioDx Molecular Detector
- Rapid COVID-19 test maker Anavasi Diagnostics lands funding from National Institutes of Health
- Medtech Startups: Sirona Medical, Proov, Anavasi Diagnostics & more
- Molecular Diagnostic System Aims to Provide PCR Lab in the Palm of Your Hand for Diagnosing COVID-19 in 30 Minutes