Robust Product Risk Analysis Templates
Download a copy of all templates and sample documents referenced on our Robust Product Risk Analysis webpage where we describe key elements of the process, clarify terminology, and provide best practices for performing a risk analysis of a medical device based upon the internationally recognized standard ISO 14971.
Full Downloadable Whitepaper in PDF format:
11 Steps to Performing a Robust Product Risk Analysis Whitepaper


Templates and Sample Documents Available in One Download:
Templates
Sample Documents
- Sample Preliminary Hazards Analysis
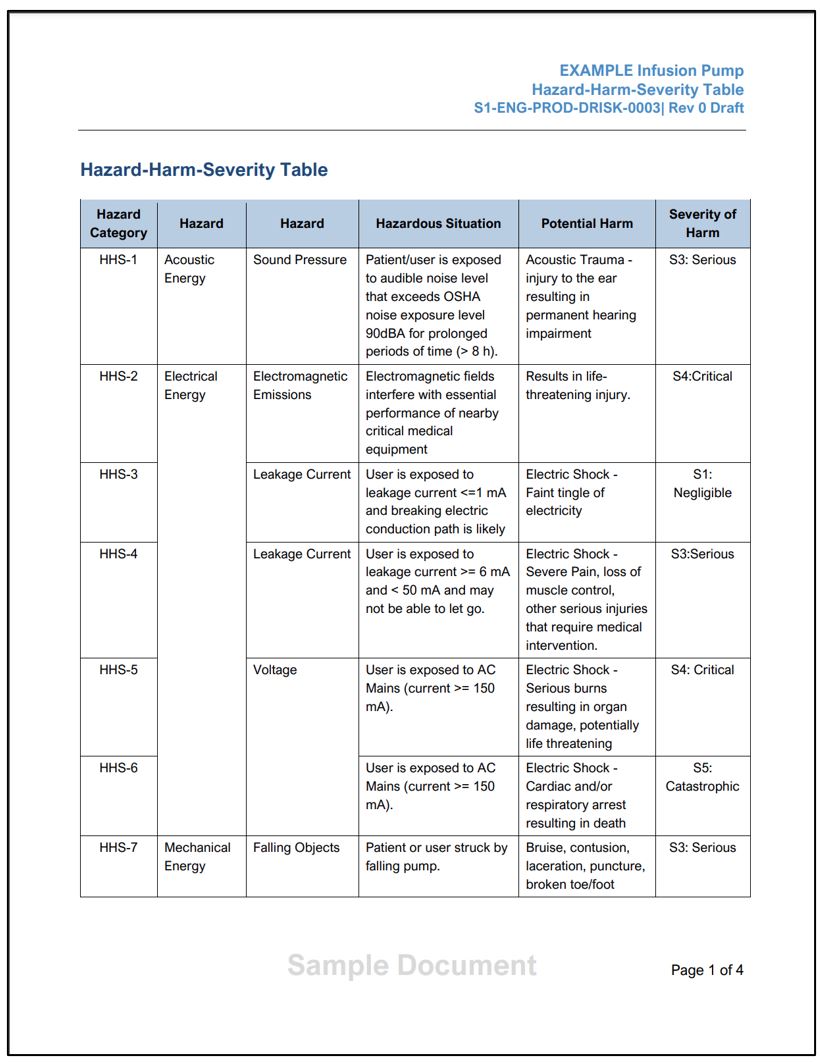
- Sample Hazard-Harm-Severity Table
- Sample Product Risk Analysis
Please contact us if you have any questions or need any help with your product development needs!
